* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Molar Mass - Science With Horne
Survey
Document related concepts
Host–guest chemistry wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Brownian motion wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Depletion force wikipedia , lookup
History of molecular theory wikipedia , lookup
Elementary particle wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Particle-size distribution wikipedia , lookup
Aerosol mass spectrometry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Transcript
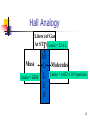
1 Moles 1 mole of a substance contains 6.022 x 1023 particles Avogadro’s number = 6.022 x 1023 So a “mole” is a number, very similar to a dozen. A dozen = 12 A mole (mol) = 6.022 x 1023 It can be found in your reference packet on page one 2 Meaning of Avogadro’s Number (The mole) The mole (abbreviated mol) is the base unit for measuring the amount of a substance. The definition of a mole comes from how many particles (atoms, in this case) there is in exactly 12 grams of Carbon-12. Through many years of experimentation, it has been confirmed that a mole of any substance has 6.022*1023 representative particles 3 4 Molar Mass or Gram Formula Mass (GFM) or Formula Weight Molar Mass = the mass of 1 mole of a substance in grams (also known as Gram Formula Weight or Formula Weight) mass given on periodic table is in amu Because amu and grams are relative we can say the masses on the periodic table are in grams To find molar mass add all the atomic masses of the elements in the compound. 5 Practice – Find the molar mass of: MgCl2 Ca(OH)2 C6H12O6 6 Moles are the central unit in chemistry. They allow us to change units between: Mass Number of particles (atoms or molecules or formula units or particles) Volume 7 Moles and Mass The conversion factor is 1 mol = Molar Mass (or GFM or FW) Convert 5.0 moles of Sulfur to grams 32.07 g S 5.0 mol S x 160 g S 1 mol S 8 Sample Problem To carry out a chemical reaction you need 3.20 moles of zinc nitrate Zn(NO3)2. What is the mass of 3.20 moles of Zn(NO3)2? 606 g Zn(NO3)2 9 Moles and Particles The conversion factor is 1 mol = 6.022 x 1023 particles (molecules, atoms, etc) Convert 2.0 moles H2O to molecules 6.022 x 10 23 mc H 2O 2.0 mol H 2 O x 1 mol H 2O 1.2 x 1024 mc H2O 10 Moles and Liters of Gas The conversion factor is 1 mol = 22.4 L It only applies to Gas at STP (standard temperature and pressure) which is 0 degrees C and 1 atm Convert 4.3 mol of O2 gas at STP to liters 22.4 L O 2 4.3 mol O 2 x 96 L O 2 1 mol O 2 11 Sample Problem A piece of marble contains 8.74 x 1023 formula units of calcium carbonate. How many moles of CaCO3 is that? 1.45 mol CaCO3 12 Hall Analogy Liters (of Gas At STP) 1 mole = 22.4 L Mass 1 mole = GFM M O L E S Molecules 1 mole = 6.022 x 1023 particles 13 Traffic Circle Analogy To get to volume, mass, or particles, you have to pass through the mole! 14 Sample Problem You need 250 grams of table sugar, or sucrose (C12H22O11), to bake a cake. How many sucrose molecules will be in the cake? 23 1 mol 6.022 x 10 mc 250 g x x 342.3 g 1 mol 4.4 x 1023 molecules C12H22O11 15 Sample Problem If you burned 4.00 x 1023 molecules of natural gas, or methane (CH4) during a laboratory experiment, what mass of methane did you burn? 10.7 g CH4 16