* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Human Herpesviruses
Survey
Document related concepts
Schistosomiasis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Neonatal infection wikipedia , lookup
Ebola virus disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Oesophagostomum wikipedia , lookup
West Nile fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Herpes simplex wikipedia , lookup
Antiviral drug wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Henipavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Transcript
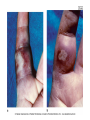
Human Herpesviruses • Human herpesviruses Three subfamilies (genome structure, tissue tropism, cytopathologic effect, site of latent infection) • Alphaherpesvirinae: Human herpesvirus 1 Herpes simplex type 1 HSV-1 Human herpesvirus 2 Herpes simplex type 2 HSV-2 Human herpesvirus 3 Varicella-zoster virus VZV • Gammaherpesvirinae Human herpesvirus 4 Epstein-Barr virus EBV Human herpesvirus 8 Kaposi’s sarcoma related virus HHV-8 • Betaherpesvirinae Human herpesvirus 5 Cytomegalovirus CMV Human herpesvirus 6Herpes lymphotropic virus HHV-6 Human herpesvirus 7 Human herpesvirus 7 HHV-7 Herpesviruses • Unique Features of Herpesviruses • Herpesviruses have large, enveloped icosadeltahedral capsids containing double-stranded DNA genomes. • Herpesviruses encode many proteins that manipulate the host cell and immune response. • Herpesviruses encode enzymes (DNA polymerase) that promote viral DNA replication and that are good targets for antiviral drugs. • DNA replication and capsid assembly occurs in the nucleus. • Virus is released by exocytosis, cell lysis, and through cell-cell bridges. • Herpesviruses can cause lytic, persistent, latent, and, for Epstein-Barr virus, immortalizing infections. • Herpesviruses are ubiquitous. • Cell-mediated immunity is required for control. Human herpesviruses They have common: • Virion morphology • Basic mode of replication • Capacity to establish latent and recurrent infections, in case of EBV immortalizing infections • Ubiquitous • Usually cause benign disease especially in children • In immunosuppressed people they cause significant morbidity and mortality e H e r p e s v i r Subfamily u s Alphaherpesvirinae e Human herpesvirus 1 s Virus Primary Target Cell Site of Latency Means of Spread Herpes simplex type 1 Mucoepithelial cells Neuron Close contact Human herpesvirus 2 Herpes simplex type 2 Mucoepithelial cells Neuron Close contact (sexually transmitted disease) Human herpesvirus 3 Varicella-zoster virus Mucoepithelial cells Neuron Respiratory and close contact Human herpesvirus 4 Epstein-Barr virus B cells and epithelial cells B cell Saliva (kissing disease) Human herpesvirus 8 Kaposi's sarcoma-related virus Lymphocyte and other cells B cell Close contact (sexual), saliva? Human herpesvirus 5 Cytomegalovirus Monocyte, lymphocyte, and epithelial cells Monocyte, lymphocyte, and ? Close contact, transfusions, tissue transplant, and congenital Human herpesvirus 6 Herpes lymphotropic virus T cells and ? T cells and ? Respiratory and close contact? Human herpesvirus 7 Human herpesvirus 7 T cells and ? T cells and ? ? Gammaherpesvirinae Betaherpesvirinae icosadeltahedral capsid and an envelope Human herpesviruses -DNA polymerase: -viral DNA replication -good target for antiviral drugs. -DNA replication and assembly:nucleus -buds from nuclear membrane, released by exocytosis and cell lysis. -lytic,persistant, latent, for EBV immortalizing infections Disease Mechanisms for Herpes Simplex Viruses Disease p a g e 5 4 4 is initiated by direct contact and depends on infected tissue (e.g., oral, genital, brain). Virus causes direct cytopathologic effects. Virus avoids antibody by cell-to-cell spread (syncytia). Virus establishes latency in neurons (hides from immune response). Virus is reactivated from latency by stress or immune suppression. Cell-mediated immunity is required for resolution with limited role for antibody. Cell-mediated immunopathologic effects contribute to symptoms. Herpes simplex virus • Two types: HSV-1 and HSV-2 • HSV can infect most types of human cells and even cells of other species. • Lytic infection of fibroblasts and epitelial cells but latent infection of neurons • The primary target cell: mucoepitelial cells • Site of latency: neurons Herpes simplex virus • Means of spread: HSV-1 close contact, HSV-2 close contact+sexual transmission! • Generally cause infection at the site of infection • HSV-1: infections above the waist • HSV-2: infections below the waist • Growth characteristics are different • HSV-2 :more potential for viremia Herpes simplex virus • Initiates infection through mucosal membranes or breaks in the skin • Virus replicates in the cells at the base of the lession and infects the innervating neurons • Travels by retrograde transport to the ganglion( trigeminal ganglion for oral HSV, sacral ganglia for genital HSV) Herpes simplex virus • Then turns to initial site of infection • May be inapparent or vesicular( vesicle fluid contains infectious virons) • Tissue damage: viral pathology+immunopathology • Heals without a scar • Latent infection occurs in neurons Herpes simplex virus Infects most types of human cells, even cells of other species. Lytic infection of fibroblasts and epitelial cells and latent infection of neurons HSV-1 binds to heparan sulfate , a proteoglycan found on the outside of many cell types Herpes simplex virus Interacts HveC (herpes virus entry mediator C) : a member of immunoglobulin protein family similar to polio virus receptor, found on most cells and neurons Penetrates by fusion During latent infection: the only region of genome to be trancribed generates latency associated transcripts(LATs) and these RNAs are not translated in protein Epidemiology • • • • • • • Virus causes lifelong infection Recurrent diseases is source of contagion Asymtomatic shedding Saliva, vaginal secretion, lesion fluid Transmitted orally, sexually, into eye, breaks in skin HSV-1 usually orally HSV-2 usually sexually Herpes simplex virus • Recurrence: stress, trauma, fever, sunlight) • The virus travels back down the nerve causing lessions at the dermatome • Recurrences are less severe and more localized • • • • HSV-1 is common 90% have antibody by 2 years of age HSV-2 occurs later in life with sexual activity Physicians,nurses,dentists at risk for infection of fingers (herpetic whitlow) • Immunocompromised people and neonates at risk of disseminated, life-threateneing disease. Clinical Syndromes • HSV-1 and HSV-2 are common human pathogens • Painful but benign manifestations and recurrent disease • A clear vesicle on an erythematous base • Pustular lesion, ulcer, crusted lesion • Sinificant morbidity and mortality on infection of eye,brain or on disseminated infection in immunosuppressed person or neonate. Clinical Syndromes • Primary herpetic gingivostomatitits • Recurrent mucocutaneous HSV(cold sores, fever blister) • Herpes pharyngitis • Herpetic keratitis: corneal damage leading to blindness • Herpetic whitlow • Eczema herpeticum • Genital herpes mostly by HSV-2 Clinical Syndromes -Herpes encephalitis: usually by HSV-1,the most common viral cause of sporodic encephalitis.Mortality is high. At all age, at any time of year -HSV meningitis: complication of genital HSV-2 -Neonatal infection: HSV-2, usually fatal Neonatal HSV • • • • • • • • HSV-2 Either during passage through genital tract Or rarely in utero Postnatally from family or hospital personel Immune system weak Disseminates to organs Baby is septic and vesicular lesions Death,or mental retardation or neurological disability Laboratory diagnosis • Cytology and histology: Tzanck smear(scraping of the base of a lesion), Papanicolaou smear or biopsy specimen • Cytopathic effects: syncytia, ballooning of cytoplasm, Cowdry A intranuclear inclusions • Direct antigen detection: immunofluorescence method or immunoperoxidase method • DNA :in situ hybridization or PCR in tissue or vesicle fluid Laboratory diagnosis • Virus isolation: CPE in 1-3 days in HeLa, Hep-2 cells, human embryonic fibroblasts and rabbit kidney cells. Isolates are identified by immunologic methods by antigen detection by IFA. • Serology:primary infection, type specific antibody by ELISA (differentiates HSV-1 and HSV-2) Approach Test/Comment Direct microscopic examination of cells from base of lesion Tzanck smear shows multinucleated giant cells and Cowdry type A inclusion bodies. Cell culture HSV replicates and causes identifiable cytopathologic effect in most cell cultures. Assay of tissue biopsy, smear, cerebrospinal fluid, or vesicular fluid for HSV antigen or genome Enzyme immunoassay, immunofluorescent stain, in situ DNA probe analysis, and polymerase chain reaction (PCR). HSV type distinction (HSV-1 vs. HSV-2) Type-specific antibody, DNA maps of restriction enzyme fragments, sodium dodecyl sulfate-gel protein patterns, DNA probe analysis, and PCR. Serology Serology is not useful except for epidemiology. Treatment • Nucleotide analogues, viral DNA polymerase inhibitors • Prevents or shortens the course of primary or recurrent disease • Can not eliminate latent infection Treatment • • • • • • • Acyclovir Penciclovir Valacyclovir Famciclovir Adenosine arabinoside Iododeoxyuridine Trifluridine FDA-Approved Antiviral Treatments for Herpesvirus Infections T h e Herpes Simplex 1 and 2 Acyclovir Penciclovir Valacyclovir Famciclovir Adenosine arabinoside Iododeoxyuridine Trifluridine Varicella-Zoster Virus Acyclovir Famciclovir Valacyclovir Varicella-zoster immune globulin Zoster immune plasma Live vaccine Epstein-Barr Virus None Cytomegalovirus Ganciclovir* Valganciclovir* Foscarnet* Cidofovir* Pregnant women • Active genital HSV • Asymtomatic shedding • Such transmission can be prevented by cesarean section • No vaccine available yet. Varicella-Zoster • • • • • Chickenpox(varicella) With recurrence :herpes zoster-shingles:zona Primary target cell: mucoepitelial cell Site of latency: neuron Means of spread: respiratory and close contact • Viremia occurs after local replication :skin lessions over the entire body Disease Mechanisms of Varicella-Zoster Virus (VZV) Initial p a g e 5 5 0 replication is in the respiratory tract. VZV infects epithelial cells, fibroblasts, T cells, and neurons. VZV can form syncytia and spread directly from cell to cell. Virus is spread by viremia to skin and causes lesions in successive crops. VZV can escape antibody clearance, and cell-mediated immune response is essential to control infection. Disseminated, life-threatening disease can occur in immunocompromised people. Virus establishes latent infection of neurons, usually dorsal root and cranial nerve ganglia. Herpes zoster is a recurrent disease; it results from virus replication along the entire dermatome. Herpes zoster may result from depression of cell-mediated immunity and other mechanisms of viral activation. Varicella-Zoster • Primary VZV infection: mucosa of respiratory tract • Viremia • Reticuloendotelial system,liver,spleen • 11-13 days later secondary viremia • Virus is spread through the body and skin=rash+fever+systemic symptoms Varicella-Zoster • Latent in dorsal root or cranial nerve ganglia after primary infection • Reactivates in older adults and in patients with impaired immunity. • On reactivation : a vezicular rash along the entire dermatome • Children and leukemia: VZV more serious and more disseminated disease Varicella-Zoster • Extremely communicable • Rates of infection exceeds 90% among household contact • Contagious before and during symptoms. • HZ develops in 10-20% of people infected with VZV and contains viable virus. Varicella(Chickenpox) • Five classic childhood exanthems: chickenpox, measles, roseola, fifth disease,rubella • Mild childhood disease • Fever+maculopapular rash • 14 days incubation • All stages of skin lesion (vesicle, pustular, crust) • More severe on trunk even on scalp • Bacterial superinfection Varicella(Chickenpox) • Severe in adults (interstitial pneumonia) • Severe in neonates and immunocompromised. Herpes-zoster • Recurrence of latent varicella • Severe pain • Rash limited to the dermatome Varicella-Zoster • • • • • • • Laboratory diagnosis: Cytology Virus isolation: difficult Serology Treatment: ACV,famciclovir and valacyclovir Prophylaxis: VZIG:varicella-zoster immunoglobulin:immunosuppressed patients • A live attenuated vaccine(Oka strain) Epstein-Barr Virus • Heterophile antibody-positive infectious mononucleosis • Chronic disease • Associated with endemic Burkitt’s lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, B-cell lymphomas in patients with acquired or congenital immunodeficiencies. • Hairy oral leukoplakia • Mitogen for B cells and immortalizes them Epstein-Barr Virus Gammaherpesvirinae: Primary target cell: B cells and epitelial cells Site of latency: B cell Means of spread: saliva (kissing disease) Limited host range and tissue tropism: receptor for C3d component of the complement system (CR2 or CD21) which is expressed on B cells of humans and some epitelial cells of oro- and nasopharynx. • • • • Select viral genes are expressed, depending on the state of the B cell; they include Epstein-Barr nuclear antigens (EBNAs) 1, 2, 3A, 3B, and 3C; latent proteins (LPs); latent membrane proteins (LMPs) 1 and 2 The EBNAs and LPs are DNA-binding proteins that are essential for establishing and maintaining the infection (EBNA-1), immortalization (EBNA-2), and other purposes. The LMPs are membrane proteins with oncogene-like activity. These proteins stimulate the growth of and immortalize the B cell. EBV establishes latency in memory B cells in which only the EBNA-1 and LMP-2 are expressed, maintaining the genome in the cells but with minimal potential for immune recognition of the infected cell. Disease Mechanisms of Epstein-Barr Virus •Virus in saliva initiates infection of oral epithelia and spreads to B cells in lymphatic tissue. •There is productive infection of epithelial and B cells. •Virus promotes growth of B cells (immortalizes). •T cells kill and limit B-cell outgrowth. T cells are required for controlling infection. Antibody role is limited. •EBV establishes latency in memory B cells and is reactivated when the B cell is activated. •T-cell response (lymphocytosis) contributes to symptoms of infectious mononucleosis. •There is causative association with lymphoma in immunosuppressed people and African children living in malarial regions (African Burkitt's lymphoma) and with nasopharyngeal carcinoma in China. EBV •The diseases of EBV result from either an overactive immune response (infectious mononucleosis) or •the lack of effective immune control (lymphoma and hairy cell leukoplakia). Epstein-Barr Virus EBV activate B-cell growth and prevents apoptosis(programmed cell death) T cell response (lymphocytosis) :atypical Lymphocytosis:Downey cells account for 1080% of total white blood cells during the second week Lymph glands,spleen and liver swells Mild in children Epstein-Barr Virus Virus causes lifelong infection May cause aymptomatic shedding Recurrent disease is a cause of contagion Children:mild or asmptomatic Teenagers and adults: infectious mononucleosis Immunocompromised people: at high risk of for life threatening neoplastic disease Diagnosis of Epstein-Barr Virus 1.Symptoms a.Mild headache, fatigue, fever b.Triad: Lymphadenopathy, splenomegaly, exudative pharyngitis c.Other: Hepatitis, ampicillin-induced rash 2.Complete blood cell count a.Hyperplasia b.Atypical lymphocytes (Downey cells) (T cells) 3.Heterophile antibody (transient) 4.EBV-antigen specific antibody • post-transplant lymphoproliferative disease • Many Hodgkin's lymphomas can also be attributed to EBV • Hairy Oral Leukoplakia EBV-associated neoplasms • • • • Geographic distribution Co-factor? Endemic Burkett’s lymphoma: Africa:malaria Nasopharyngeal carcinoma: China Laboratory diagnosis • Heterophile antibody: results from nonspecific activation of B cells by EBV • IgM antibody recognizes Paul-Bunnell antigen on sheep, horse and bovine erythrocytes not on guinea pig kidney cells • Detected at the end of first week , lasts for several months • Monotest, ELISA: specific antibodies • VCA-IgM, antibody to early antigen (EA): recent infection • VCA-IgG, EBNA: previous infection Cytomegalovirus(CMV) • Betaherpesvirnae: lymphotropic • Primary target cell: monocyte, lymphocte, epitelial cell • Site of latency: monocyte, lymphocyte and? • Means of spread: close contact, transfusions, tissue transplant and congenital Sources of infection • Neonate: transplacental transmission, intrauterine infection, cervical secretion • Baby or child: body secretions, breast milk, saliva, tears, urine • Adult: sexual transmission(semen), blood transfusion, organ graft Clinical findings • Predominant presentation: asymptomatic • Neonates: deafness, mental retardation • Immunosuppressed patients: disseminated dissease, severe disease (pneumonia, retinitis, colitis, esophagitis) CMV • the most common viral cause of congenital defects • particularly important as an opportunistic pathogen in immunocompromised patients Congenital infection • An important cause of congenital disease • Serious birth defects is high if primary infection occurs during pregnancy • Microcephaly, intracerebral calcification,hepatosplenomegaly,rash(cytomegalic inclusion disease), unilateral or bilateral hearing loss, mental retardation • CMV in the urine in the first week (culture,PCR) CMV immunsupressed patients • CMV disease of the lung (pneumonia and pneumonitis) is a common outcome in immunosuppressed patients • Retinitis (%10-15 AIDS patients) • Interstitial pneumonia and encephalitis • colitis or esophagitis may develop in as many as 10% of patients with AIDS • May be related with GVHR after bone marrow transplantation o v i r u s S y n d r o m e s Immunosuppressed Patients Tissue Children/Adults Predominan t presentation Asymptomatic Disseminated disease, severe disease Eyes - Chorioretinitis Lungs - Pneumonia, pneumonitis Gastrointesti nal tract - Esophagitis, colitis Nervous system Polyneuritis, myelitis Meningitis and encephalitis, myelitis Lymphoid system Mononucleosis syndrome, posttransfusion syndrome Leukopenia, lymphocytosis Major organs Carditis,* hepatitis* Hepatitis Neonates Deafness, intracerebral calcification, microcephaly, mental retardation - . Epidemiology of Cytomegalovirus Infection p a g e Disease/Viral Factors •Virus causes lifelong infection. •Recurrent disease is source of contagion. •Virus may cause asymptomatic shedding. Transmission •Transmission occurs via blood, organ transplants, and all secretions (urine, saliva, semen, cervical secretions, breast milk, and tears). •Virus is transmitted orally and sexually, in blood transfusions, in tissue transplants, in utero, at birth, and by nursing. Geography/Season •Virus is found worldwide. •There is no seasonal incidence. Who Is at Risk? •Babies. •Babies of mothers who experience seroconversion during term: At high risk for congenital defects. •Sexually active people. •Blood and organ recipients. •Burn victims. •Immunocompromised people: Symptomatic and recurrent disease. Modes of Control •Antiviral drugs are available for patients with acquired immune deficiency syndrome. •Screening potential blood and organ donors for cytomegalovirus reduces transmission of virus. Laboratory tests • Cytology and histology: ‘OWL’s eye’ inclusion body basophilic intranuclear:Urine not so sensitive • Antigen in peripheral leucocytes • DNA by PCR • Cell culture: Human diploid fibroblasts • Serology: primary infection Treatment • • • • • Ganciclovir Foscarnet Valganciclovir Cidofovir No vaccine Other mononucleosis causes • • • • EBV CMV HIV Toxoplasma gondii Human herpesvirus 6 • • • • • • • Betaherpesvirinae Lymphotropic , ubiquitous Primary target cell: T cells and ? Site of latency: T cells and ? Means of spread: Respiratory, close contact Exanthema subitum: roseola A mononucleosis syndrome and lympadenopathy Exanthema subitum • High fever+rash for 24-48 hours • Incubation 4-7 days • Recovery without complication Human herpesvirus 8 • HHV-8 DNA sequences were discovered by PCR in biopsy specimens of • Kaposi’s sarcoma (characteristic opportunistic diseases associated with AIDS) • Primary effusion lymphoma (rare type of Bcell lymphoma) • Multicentric Castleman’s disease Human herpesvirus 8 • Kaposi’s sarcoma-related virus • Primary target cell: Lymphocyte and other cells • Site of latency:? • Means of spread: close contact, sexual, saliva? • Limited to certain geographic areas (Italy, Greece, Africa) and AIDS Human herpesvirus 8 • Laboratory diagnosis: • Serology: specific antibodies:IFA IgG,IgM • HHV-8DNA by PCR Herpesvirus simiae(B virus) • • • • Asian monkeys Bites, saliva Encephalopathy in humans fatal