* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 21.8 The Citric Acid Cycle
Survey
Document related concepts
Radical (chemistry) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
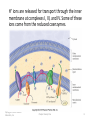
21.8 The Citric Acid Cycle • The carbon atoms from the first two stages of catabolism are carried into the third stage as acetyl groups bonded to coenzyme A. • Like the phosphoryl groups in ATP molecules, the acetyl groups in acetyl-SCoA molecules are readily removed in an energy-releasing hydrolysis reaction. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 1 • Oxidation of two carbons to give CO2 and transfer of energy to reduced coenzymes occurs in the citric acid cycle, also known as the tricarboxylic acid cycle (TCA) or Krebs cycle (after Hans Krebs, who unraveled its complexities in 1937). ► The citric acid cycle is a closed loop of reactions in which the product of the final step which has four carbon atoms, is the reactant in the first step. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 2 • The net result of the citric acid cycle is: – Production of four reduced coenzyme molecules, 3 NADH and 1 FADH2 – Conversion of an acetyl group to two CO2 molecules – Production of one energy-rich molecule (GTP) • ADP acts as an allosteric activator for the enzyme for Step 3. NADH acts as an inhibitor of the enzyme for Step 3. • By such feedback mechanisms, the cycle is activated when energy is needed and inhibited when energy is in good supply. • The eight steps of the citric acid cycle are shown in greater detail on the next slide. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 3 Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 4 21.9 The Electron-Transport Chain and ATP Production • At the conclusion of the citric acid cycle, the reduced coenzymes formed in the cycle are ready to donate their energy to making additional ATP • Hydrogen and electrons from NADH and FADH2 enter the electron-transport chain at enzyme complexes I and II, respectively. • The enzyme for Step 6 of the citric acid cycle is part of complex II. FADH2 produced there does not leave complex II. Instead it is immediately oxidized there by reaction with coenzyme Q. • Following formation of the mobile coenzyme Q, reductions occur when electrons are transferred. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 5 ► Coenzyme Q is also known as ubiquinone because of its widespread occurrence and because its ring structure with the two ketone groups is a quinone. • Electrons are passed from weaker to increasingly stronger oxidizing agents, with energy released at each transfer. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 6 Other important electron acceptors are various cytochromes, which are proteins that contain heme groups in which the iron cycles between Fe+2 and Fe+3 and proteins with iron–sulfur groups in which the iron also cycles between Fe+2 and Fe+3 . Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 7 H+ ions are released for transport through the inner membrane at complexes I, III, and IV. Some of these ions come from the reduced coenzymes. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 8 • The H+ concentration difference creates a potential energy difference across the two sides of the inner membrane (like the energy difference between water at the top and bottom of the waterfall). • The maintenance of this concentration gradient across the membrane is crucial—it is the mechanism by which energy for ATP formation is made available. • ATP synthase: The enzyme complex in the inner mitochondrial membrane at which H+ ions cross the membrane and ATP is synthesized from ADP. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 9 • Oxidative phosphorylation: The synthesis of ATP from ADP using energy released in the electron transport chain. • Each of the enzyme complexes I–IV contains several electron carriers. • In the last step of the chain, electrons combine with oxygen that we breathe and H+ ions from the surroundings to produce water. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 10 21.10 Harmful Oxygen By-Products and Antioxidant Vitamins • More than 90% of the oxygen we breathe is used in electron transport– ATP synthesis reactions. • In these and other oxygen-consuming redox reactions, the product may not be water, but one or more of three highly reactive species. • The superoxide ion, ·O2- , and the hydroxyl free radical, ·OH, can grab an electron from a bond in another molecule, which results in breaking that bond. The third oxygen by-product is hydrogen peroxide, H2O2 , a relatively strong oxidizing agent. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 11 Conditions that can enhance production of these three reactive oxygen species are represented in the drawing below. Some causes are environmental, such as exposure to smog or radiation. Others are physiological, including aging and inflammation. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 12 • Our protection against harmful oxygen species is provided by superoxide dismutase and catalase, which are among the fastest-acting enzymes. • These and other enzymes are active inside cells where oxygen by-products are constantly generated. It is estimated that 1 in 50 of the harmful oxygen species escapes destruction inside a cell. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 13 • Protection is also provided by the antioxidant vitamins E, C, and A , all of which disarm free radicals by bonding with them. • Vitamin E is fat-soluble, and its major function is to protect cell membranes from potential damage. Copyright © 2010 Pearson Education, Inc. Chapter Twenty One 14