* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Paper chromatography
Spinodal decomposition wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Colloidal crystal wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Solvent models wikipedia , lookup
Implicit solvation wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Phase transition wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Monolithic HPLC column wikipedia , lookup
Ion chromatography wikipedia , lookup
Gas chromatography wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Micellar liquid chromatography wikipedia , lookup
Countercurrent chromatography wikipedia , lookup
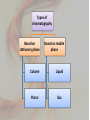
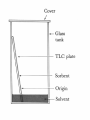
• • Mikhail Tswett, Russian, 1872-1919 In 1906 Tswett used to chromatography to separate plant pigments, He called the new technique chromatography because the result of the analysis was • Most materials in our surroundings are mixtures of two or more components. Mixtures are either homogeneous or heterogeneous: • • • Homogeneous mixtures are uniform in composition, but heterogeneous mixtures are not. Air is a homogeneous mixture and oil in water is a heterogeneous mixture. Homogeneous and heterogeneous mixtures can be separated into their components by several physical methods. • The choice of separation techniques is based on the type of mixture and difference in the chemical properties of the constituents of a mixture. • Various types of separation processes but we will concern on chromatography in this lecture • Chromatography is a widely used experimental technique for the separation of a mixture of compounds into its individual components. • The word chromatography means "separation of colors" but today chromatography is used for both colored and colorless substances. • Chromatography is a physical method of separation in which the components to be separated are distributed between two phases • one of which is stationary stationary phase, while the other the mobile phase moves through it in a definite direction. • Works by allowing the molecules present in the mixture to distribute themselves between a stationary and a mobile medium. Chromatography Electrophoresis Difference in rate of movement of a solute through a stationary phase Difference in migration rate in an electrical field gradient can be used for liquid, solid and gaseous compounds is generally carried out on liquid and solid compounds partition coefficient of the element is used to carry out the investigations and separation the electrical property of the element is used Affinity: natural attraction or force between things. Adsorption: refers to the adhesion or stickyness of a substance to the surface of another substance, as opposed to the term “absorption” which refers to a substance penetrating into the inner structure of another substance. https://www.youtube.com/watch?v=djIzXvwIz 5U • The separation process is based on the fact that porous solids adsorbs different substances to different extremes depending upon their polarity. • A mixture to be separated is first applied to an immovable porous solid (like paper, or alumina, or fine silica sand) called the stationary phase. • The components of the mixture then get “washed” along the porous solid by the flow of a solvent called the mobile phase. Each component of a mixture to be separated will be attracted differently to the porous stationary phase depending on its polarity and the polarity of the stationary phase chosen. Remember that “Like dissolve Like”. If the stationary phase is polar then polar components will be attracted or stick more to it but non-polar components will move across the surface easily. If the stationary phase is nonpolar then nonpolar components will be more attracted to it and the polar compounds will move along more quickly. • Chromatography involves the sample being dissolved in a particular solvent called mobile phase. • The mobile phase may be a gas or liquid. • The mobile phase is then passed through another phase called stationary phase. • The stationary phase may be a solid packed in a glass plate or a piece of chromatography paper. Can separate complex mixtures with great precision. Even very similar components, such as proteins that may only vary by a single amino acid. Can purify basically any soluble or volatile substance if the right adsorbent material, carrier fluid, and operating conditions are employed. Exact quantitative analysis is done even from trace compounds. Small material consumption. The quantization has a broad linearity range. Analyses of several compound can be done during one run. Chromatography is a fast analysis method. Well established instrumentation with high level automation is commercially available. Stationary Phase Separation Mobile Phase Mixture Components Components Affinity to Stationary Phase Affinity to Mobile Phase • Analyze Blue ---------------- Insoluble in Mobile Phase • Identify Black Red Yellow • Purify •Quantify Types of chromatography Based on stationary phase Based on mobile phase Column Liquid Planar Gas 1- Liquid chromatography: mobile phase is a liquid. (LLC, LSC). 2- Gas chromatography : mobile phase is a gas. (GSC, GLC). Planar chromatography: A separation technique in which the stationary phase serves as a plane. – The plane can be either a paper (paper chromatography) or a layer of solid particles sorbent (silica gel, cellulose, aluminum oxide, ion exchange resin) spread on a support such as a glass- or a plastic- plate (thin layer chromatography). THIS allowing to carry out qualitative and quantitative analysis of chemical components in complex mixtures. 1- Thin layer chromatography (TLC): the stationary phase is a thin layer supported on glass, plastic or aluminium plates. 2- Paper chromatography (PC): the stationary phase is a thin film of liquid supported on an inert support. Column chromatography (CC): stationary phase is packed in a glass column. For qualitative analysis the different mobilities of substances are used, the distances passed by different substances are different. The distance between the starting line and the center of the spot of substance characterizes the substance. Retention factor, RF, provides better way to indentify substances . Identification of Unknown Compounds For your initial analysis, spot your unknown and the colored standard compounds on one plate. Be sure to note which spot corresponds to which compound. For quantitative determination the intensity of the spot is used: the bigger the amount of substance in the mixture, the more intensive is the spot. Also the size of the spot can give quantitative information – the bigger the spot, the bigger the content of this compound in the mixture. Intensity of the spots is evaluated by comparing with the intensities of analyte spots with known amounts visually or using densitometer. • Paper chromatography uses paper as the stationary phase and a liquid solvent as the mobile phase. • In paper chromatography, the sample is placed on a spot on the paper and the paper is carefully dipped into a solvent. • The solvent rises up the paper due to capillary action and the components of the mixture rise up at different rates and thus are separated from one another. Capillary Action: the movement of liquid within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. Cohesion, phenomenon of intermolecular forces holding particles of a substance together. Adhesion, attraction between the surfaces of two bodies. The liquid is able to move up the filter paper because its attraction to itself is stronger than the force of gravity. Solubility: the degree to which a material (solute) dissolves into a solvent. Solutes dissolve into solvents that have similar properties. (Like dissolves like) This allows different solutes to be separated by different combinations of solvents. Separation of components depends on both their solubility in the mobile phase and their differential affinity to the mobile phase and the stationary phase. • Thin layer chromatography is a technique used to separate and identify compounds of interest. • A TLC plate is made up of a thin layer of silica adhered to glass or aluminum for support. • The silica gel acts as the stationary phase and the solvent mixture acts as the mobile phase. • In the ideal solvent system the compounds of interest are soluble to different degrees. Separation results from the partition equilibrium of the components in the mixture. Different compounds in sample mixture travel different distances according to how strongly they interact with the stationary phase as compared to the mobile phase. In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will be carried farther up the plate than others. This liquid, or the eluent, is the mobile phase, and it slowly rises up the TLC plate by capillary action. The specific Retention factor (Rf) of each chemical can be used to aid in the identification of an unknown substance. • Rf values can be calculated and compared with the reference values to identify the amino acids. • Highly polar molecules interact fairly strongly with the polar SiOH groups at the surface of these adsorbents, and will tend to stick or adsorb onto the fine particles of the adsorbent while weakly polar molecules are held less tightly. • Weakly polar molecules generally tend to move through the adsorbent more rapidly than the polar species. Roughly, the compounds follow the elution order given above. • Different compound in sample mixture travel different distance according to how strongly they interact with the stationary phase as compared to mobile phase. ( ) • The separation depends on several factors: 1. solubility: the more soluble a compound is in a solvent, the faster it will move up the plate.(polarity of mobile phase) 2. attractions between the compound and the silica, the more the compound interacts with silica, the lesser it moves, (. activity of stationary phase) 3. structure of substrate silica gel • Silica gel is a form of silicon dioxide (silica). The silicon atoms are joined via oxygen atoms in a giant covalent structure. However, at the surface of the silica gel, the silicon atoms are attached to -OH groups. • The surface of the silica gel is very polar and, because of the -OH groups, can form hydrogen bonds with suitable compounds around it. The other commonly used stationary phase is alumina aluminium oxide. The aluminium atoms on the surface of this also have -OH groups attached. • TLC is a useful technique because it is relatively quick and requires small quantities of material. o Eluent: The mobile phase (usually for solvents) o Elution: Motion of the mobile phase through the stationary phase o Elution time: The time taken for a solute to pass through the system. A solute with a short elution time travels through the stationary phase rapidly, i.e. it elutes fast. o Mobile phase: The part of the chromatography system that is mobile. Commonly a solvent mixture (as in column chromatography or thin layer chromatography or a gas (as in gas chromatography). • Eluent. [Mix n-butanol, acetic acid (purity 98 – 100 %) and distilled water in volume ratio 5:1:5. Stir for 10 minutes, then let the layers separate. Use upper layer as eluent]. • Developing Solution: Dissolve 0.3 g of ninhydrin in 100 ml n-butanol. Add 3 ml of glacial acetic acid. • • 0.02 M solutions of different amino acids (e.g. leucine, methionine, alanine and serine) in H2Odd. Chromatographic paper • Elution chamber, Glass capillaries for spotting the samples. • Drying oven at ~ 60° C. 1. Rubber gloves must be used during this work to avoid contamination of chromatographic paper with amino acids from skin, and for protecting skin from solvents and ninhydrin while working with the sprayer or sprayed paper. 2. While the paper is being prepared for chromatographic analysis it should be kept on a piece of filter paper. 3. Mark the starting line to the paper - 8-9 mm from the edge of the plate with graphite pencil (very slight line!). Also mark the locations where the samples will be spotted. The distance between neighboring spots should be about 8 mm and the spots should be at least 5 mm away from the paper’s edge. Usually the spot of unknown substance is applied to the center of the starting line. pencil (do not use a pen, as the dye may separate and run along with your sample), Over spotting or inadequte spacing between samples can lead to their bleeding into each other during the run. Place one end of the tube into the sample. Through capillary action the sample will be drawn into the tube. Place your finger over the other open end of the tube to prevent the sample from falling out. Hold the tube slightly above the location on the starting line that you had previously marked for spotting. Carefully allow air into the opposite end of the capillary tube by slightly raising your finger that is covering the opening. Allow only a single drop to fall on the plate -- the smaller the better. If the sample is not leaving the tube, slight tapping on the opposite end should coax out a droplet. Allow the spot to evaporate. Repeat this procedure 2-3 times. Concentration of your spotting will definitely affect your TLC run and it is a matter of experimentation to determine what will give you the best results. Repeat this procedure for the remaining samples. The plates only allow for 2-3 samples to be spotted to provide for adequate spacing between sample spots. Over spotting or inadequate spacing between samples can lead to their bleeding into each other during the run. 4. Before applying samples to the paper and filling the elution chamber fit the length of chromatographic paper with the height of elution chamber. 5. The spots of individual amino acids and sample solutions are applied to the chromatographic paper. Use separate clean and dry glass capillary for each solution. Dip the capillary into solution – some solution is drawn into the capillary. With the filled capillary touch the prepared location on chromatographic paper. The spot on the paper should not be bigger than 2-3 mm. (You can exercise spotting on a sheet of filter paper.) 6. After application of samples let the spots dry. Meanwhile measure with a graduated test-tube 5 ml of eluent into the elution chamber. Cover the chamber with lids and let the chamber atmosphere saturate with eluent vapors for at least 10 min. 7. Elution is stopped when the solvent front has traveled up the plate until 7-10 mm from the lid. 8. Remove the paper from elution chamber and place it on a sheet of filter paper. After 2-3 minutes mark the eluent front with pencil and dry the paper in oven. When the plates are removed from the chamber, quickly trace the solvent front (the highest solvent level on the plate) with a pencil. Monitor the run. The time that it takes for the solvent to rise up the plate is dependent upon your choice of solvent. Generally a run will take only a few minutes, but you must monitor the solvent front to be sure that it does not reach the end of the plate. This could result in your sample being carried to the end of the plate and an unusable TLC run. A good guideline to follow is to allow the solvent front to reach a point about 1 cm from the end of the slide. 9. When the paper is dry, take it into the fume hood and spray it with solution of ninhydrin until the paper is slightly damp. Chromatographic paper and the paper supporting it should lie at 45° angle while spraying. The chromatographic paper is again put in the drying oven (60 C) for 15 min to speed up the reactions. 10. Remove chromatographic paper from drying in the oven, draw the contours and centers of the chromatographic bands. Calculate RF values by the method described above. 11. Compare retention of standard substances and components in sample and determine which amino acids were present in the sample. 1. Adding fluorescence indicators to the sorbent layer during the process of preparation of the plates or spraying the plates with fluorescent solutions and then observing under ultraviolet lamp. 2. I2 vapor as indicator . 3.Ninhydrin spray.(Visualizing Agents for amino acids ) Development of Ruhemann’s purple from ninhydrin and amino acid. This value is constant for any given compound under a given set of conditions and can be used to identify an unknown compound. Separation of compounds is based on the competition of the solute and the mobile phase for binding places on the stationary phase. For instance, if normal phase silica gel is used as the stationary phase it can be considered polar. Given two compounds which differ in polarity, the more polar compound has a stronger interaction with the silica and is therefore more capable to dispel the mobile phase from the binding places. Consequently, the less polar compound moves higher up the plate (resulting in a higher Rf value). If the mobile phase is changed to a more polar solvent or mixture of solvents, it is more capable of dispelling solutes from the silica binding places and all compounds on the TLC plate will move higher up the plate Is this a pure substance or mixture? TLC can be used to determine whether a sample is a single substance or a mixture. A single pure substance gives a single spot. However, care should be taken since compounds of similar properties, isomers for example, may not separate. A sample that results in many spots after development is a confirmation of a mixture of different compounds. In that sense, TCL can also be used to determine the number of components in a mixture. Are these compounds identical? TLC can be used to confirm whether two or more compounds are identical or not. The compounds to be analyzed are spotted on the same plate and the plate developed. If the compounds have the same Rf value, this can be taken as an indication that they have a similar chemical nature. However, it is important to keep in mind that many chemical compounds have the same Rf and TLC cannot be used as the only analytical tool to decide on the identity of a chemical compound.