* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Reactions presentation
Survey
Document related concepts
Chemical potential wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Electrochemistry wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Transcript
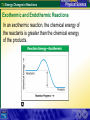
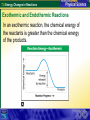
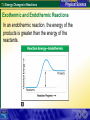
7.3 Energy Changes in Reactions Reaction of sodium and chlorine 7.3 Energy Changes in Reactions Chemical Bonds and Energy What happens to chemical bonds during a chemical reaction? Chemical reactions involve the breaking of chemical bonds in the reactants and the formation of chemical bonds in the products. 7.3 Energy Changes in Reactions Chemical Bonds and Energy The heat produced by a propane grill is a form of energy. When you write the chemical equation for the combustion of propane, you can include “heat” on the right side of the equation. C3H8 + 5O2 3CO2 + 4H2O + Heat 7.3 Energy Changes in Reactions Chemical Bonds and Energy 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions What happens to energy during a chemical reaction? During a chemical reaction, energy is either released or absorbed. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions In an exothermic reaction, the chemical energy of the reactants is greater than the chemical energy of the products. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions In an exothermic reaction, the chemical energy of the reactants is greater than the chemical energy of the products. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions Endothermic Reactions A chemical reaction that absorbs energy from its surroundings is called an endothermic reaction. In an endothermic reaction, more energy is required to break the bonds in the reactants than is released by the formation of the products. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions In an endothermic reaction, the energy of the products is greater than the energy of the reactants. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions In an endothermic reaction, the energy of the products is greater than the energy of the reactants. 7.3 Energy Changes in Reactions Exothermic and Endothermic Reactions At about 450°C, mercury(II) oxide decomposes into oxygen gas and liquid mercury. The decomposition of mercury(II) oxide is an endothermic reaction. 2HgO + 181.7 kJ 2Hg + O2