* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download What is a mixture?
Electrochemistry wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical potential wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Photopolymer wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical industry wikipedia , lookup
History of molecular theory wikipedia , lookup
Water pollution wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Water splitting wikipedia , lookup
Stoichiometry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Safety data sheet wikipedia , lookup
Homoaromaticity wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Atomic theory wikipedia , lookup
Organic chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical element wikipedia , lookup
Periodic table wikipedia , lookup
Drug discovery wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
History of chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
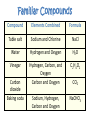
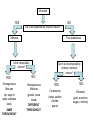
What are Elements? • An element is a pure substance that cannot be separated or broken down into simpler substances by physical or chemical means. • What are examples of elements? – Anything that is on the Periodic Table of Elements. – Examples: Gold (Au), Silicon (Si), Neon (Ne), Silver (Ag), sulfur (S) S Au Ag Fe The Periodic Table of Elements Everything that is on this table is an element. If it isn’t on this table, it isn’t an element! Pure Substances • Pure substances are substances in which there is only one type of particle. • These particles are called atoms. • The only two things that are pure substances are: 1. Elements 2. Compounds Identifying Elements • Elements are categorized by unique properties on the Periodic Table. • They are arranged in order by their number of protons. (More on this later!) • Each element has unique properties like melting point, boiling point, and whether it is metal, nonmetal or metalloid. What are compounds? • A compound is a pure substance composed of two or more elements chemically combined. • This means they were formed by a chemical reaction. Elements Compounds Familiar Compounds Compound Elements Combined Formula Table salt Sodium and Chlorine NaCl Water Hydrogen and Oxygen H2O Vinegar Hydrogen, Carbon, and Oxygen Carbon and Oxygen C2H4O2 Sodium, Hydrogen, Carbon and Oxygen NaCHO3 Carbon dioxide Baking soda CO2 Forming a Compound • Compounds are formed by combining two or more elements. – Elements are “stuck together” by chemical bonds – When this happens, new properties are formed; the elements lose their original properties. – You end up with one new thing! ELEMENTS MAKE COMPOUNDS!! Example: Formation of NaCl + Sodium is a soft, silvery white metal that reacts violently with water. Chlorine is a poisonous, greenish-yellow gas. Sodium Chloride, or table salt, is a white solid. It dissolves easily in water and is safe to eat. **Compounds have properties that differ from those of the elements that form it!** How are compounds separated? • Compounds are broken apart by breaking chemical bonds. – You separate them by forcing another chemical reaction to happen – CHEMICAL CHANGE!!!! – Add heat, electricity, another compound or element as a chemical reaction • Remember compounds are specific recipes! They have a fixed ratio! Breaking Chemical Bonds What is a mixture? • A mixture is when 2 or more substances are combined but do not chemically react. • THE SUBSTANCES KEEP THEIR OWN PROPERITES!! • We say that we MIX to form them. Examples of Mixtures 1. 2. 3. 4. Iced tea powder and water Granite Milk Oil and vinegar 3 Properties of a Mixture 1. It is NOT a chemical change 2. It can be separated by physical means 3. Ratio of each substance does NOT matter Separation of a Mixture 1. 2. 3. 4. 5. Pick apart or straining Evaporation Filter Centrifuge Distillation (boiling the solvent to separate it from the solute) 6. Magnetism (like iron) Separating a mixture is a PHYSICAL CHANGE because there are no chemical reactions or changes - parts keep their properties!! Types of Mixtures Homogeneous (Homo = same) The mixture appears to be the SAME throughout Heterogeneous (Hetero = different) The mixture appears to be DIFFERENT throughout Examples HOMOGENEOUS Milk Stainless steel Oil Brass (Cu + Zn) HETEROGENEOUS Oil & vinegar Concrete Soil Pizza, Cereal All matter YES NO Can it be separated by physical means? Mixtures Pure substances Is the composition uniform? YES: Can it be decomposed by ordinary chemical means? NO: Homogeneous Mixtures Heterogeneous Mixtures (air, sugar in water, stainless steel) (granite, wood, blood) SAME THROUGHOUT DIFFERENT THROUGHOUT YES: NO: Compounds Elements (water, sodium chloride, quartz) (gold, aluminum, oxygen, chlorine)