* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter13
Survey
Document related concepts
Physical organic chemistry wikipedia , lookup
Rubber elasticity wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Marcus theory wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
George S. Hammond wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Glass transition wikipedia , lookup
Temperature wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Transition state theory wikipedia , lookup
Thermodynamics wikipedia , lookup
Gibbs paradox wikipedia , lookup
Transcript
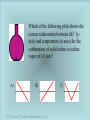
Chapter 13 Thermodynamics: Spontaneous Processes, Entropy, and Free Energy Chapter 13: Entropy and Free Energy In chapter 13 the question of why some chemical (or physical) processes are spontaneous is asked. R→P The answer is found within the 2nd Law of thermodynamics. Spontaneous processes occur due to and increase in the total entropy (S), i.e. DS > 0 for the system plus the surrounding. For example, exothermic chemical reactions are often spontaneous because of increases in disorder of the surrounding caused by the release of heat. DSuniverse = DSsystem + DSsurrounding Entropy is also a measure of the unavailability of the system’s energy to do work. Consider that heat and work are both forms of energy. Which is the more ordered form? Entropy (S) is defined quantitatively by the amount of heat absorbed (isothermally) by the system divided by the absolute temperature DS = Dq/T (Joule/K) For a glass of ice melting the heat from the room is transferred to the ice until the temperatures are equal. The dispersal of energy…warmer to cooler result always results in an increase in entropy. Maxwell’s Demon…a gedanken experiment. Two containers are filled with the same gas at equal temperatures. When a faster than average molecule flies near the door the demon opens it. Over time the average temperature will increase in one side and decrease in the other…driving the system out of equilibrium. ENTROPY AND WHY ENDOTHERMIC PROCESSES OCCUR. Entropy (S°) is a measure of randomness or disorder in a system or its surrounding. Spontaneous processes are accompanied by a net increase in the entropy of the universe. This statement is know as the 2nd Law of thermodynamics. DSuniverse = DSsystem + DSsurrounding So that… DSuniverse = DSsystem + DSsurrounding > O Third Law of Thermodynamics • Third Law of Thermodynamics - the entropy of a perfect crystal is zero at absolute zero. S is explicitly known (=0) at 0 K, S values at other temps can be calculated. • Absolute entropy is the entropy change of a substance taken from S = 0 (at T = 0 K) to some other temperature. • Standard molar entropy (So) is the absolute entropy of 1 mole of a substance in its standard state. The 3rd Law of Thermodynamics defines the entropy of a perfect crystal, at zero Kelvin to be equal to zero. Graphite has a higher standard molar entropy than diamond. Why might that be? S° = 2.4 J/mol K S° = 5.7 J/mol K The entropy of a substance like water is linked to the motion (i.e. the temperature) of the molecules Select Standard Molar Entropy Values (1 atm, 298 K) Formula So (J/(mol•K) Formula Name So (J/(mol•K) Br2(g) 245.5 CH4(g) methane 186.2 Br2(l) 152.2 CH3CH3(g) Ethane 229.5 Cdiamond(s) 2.4 CH3OH(g) Methanol 239.7 Cgraphite(s) 5.7 CH3OH(l) CO(g) 197.7 CH3CH2OH(g) CO2(g) 213.8 CH3CH2OH(l) H2(g) 130.6 CH3CH2CH3(g) Propane 269.9 N2(g) 191.5 CH3(CH2)2CH3(g) n-Butane 310.0 O2(g) 205.0 CH3(CH2)2CH3(l) H2O(g) 188.8 C6H6(g) H2O(l) 69.9 C6H6(l) NH3(g) 192.3 C12H22O11(s) 126.8 Ethanol 282.6 160.7 231.0 Benzene 269.2 172.8 Sucrose 360.2 Molecular entropy is also linked to the complexity of molecular structure, i.e. the internal motions of the molecules and molar mass. Standard Molar Entropies (298K, 1 bar) Compound (g) S° (J/mol۰K) He 126 Ar 155 CH4 186 C2H6 230 C3H8 270 C4H10 310 Standard Molar Entropies values increase with mass (number of electrons) which leads to more accessible energy levels (microstates) Problem Rank the compounds in each of the following groups in order of increasing standard molar entropy, S°. a. CH4(g), C2H6(g), and C2H8(g) b. CCl4(l), CHCl3(l), and CH2Cl2(l) c. CO2(s), CO2(g), and CS2(g) Find the compounds in Appendix 4 and compare the actual values. Problem 36 Predict the sign of DS for each of the following processes. a. Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq) b. C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l) c. N2O5(g) → NO2(g) + NO3(g) Entropy Calculations We can calculate the entropy of a chemical reaction using standard molar entropies and the stoichiometry of the balanced chemical equation. DSrxn = SnS°[products] – SmS°[reactants] Use the standard molar entropies in Appendix 4 to calculate the DS° value for the following reaction. H2S(g) + 3/2 O2(g) ↔ H2O(g) + SO2(g) Free Energy The magnitudes and signs of DSsys and DSsurr determine the magnitude and sign of DSuni. If DSuni > 0 then the reaction is spontaneous Free Energy (DGsys) is the maximum amount of energy available to do work. For DGsys < 0 (i.e. negative) the reaction is spontaneous (exergonic). DGsys > 0 (i.e. positive) the reaction is not spontaneous (endergonic) DGsys = 0 ; the system is at Equilibrium Since DGsys depends on temperature, some reactions will become spontaneous as the temperature of the system increases or decreases. Gibbs Free Energy Function DGsys = DHsys - TDSsys Changes in entropy, enthalpy and free energy for ice melting DG<0 Calculate and compare values for DSsys and Dssurr for the reaction: H2(g) + ½ O2(g) → H2O(g) Free energy (we now see) is the maximum amount of energy available to do work. It can also be calculated using Standard Free Energy of Formation Values (Appendix 4) DGrxn = SnGf°[products] – SmGf°[reactants] Use the values in Appendix 4 to calculate the DG°rxn for the following reaction: H2S(g) + 3/2 O2(g) ↔ H2O(g) + SO2(g) Driving Forces for Spontaneous Chemical Processes 1. The formation of low energy products (exothermic processes; DH < 0) 2. The formation of products that have greater entropy than the reactants (DS > 0). • The free energy (G) relates enthalpy, entropy, and temperature for a process. G = H - TS or DG = DH - TDS Effects of DH, DS, and T on DG* DH DS DG Spontaneity <0 >0 Always <0 Always Spontaneous <0 <0 <0 at low temp Spontaneous at Low Temperatures >0 >0 <0 at high temp Spontaneous at High Temperatures >0 <0 Always >0 Never Spontaneous DG = DH - TDS Show that hydrogen cyanide (HCN) is a gas at 25°C by estimating its normal boiling point from the following data: DH°f, kJ/mol S°, J/(mol · K) HCN(l) 108.9 113 HCN(g) 135.4 202 Spontaneous Processes • In thermodynamics, a spontaneous process is one that proceeds in a given direction without outside intervention. Spontaneous process can take forever! • A non-spontaneous process only occurs for as long as energy is continually added to the system. Thermodynamics • The second law of thermodynamics states that the total entropy of the universe increases in any spontaneous process. • Entropy (S) is a measure of the distribution of energy in a system at a specific temperature. Entropy and Microstates • Quantum mechanics teaches that energy is not continuous at the atomic scale; only certain levels of energy are possible. • The motion of molecules is quantized, which means different states are separated by specific energies. • An energy state, also called an energy level, is an allowed value of energy. • A microstate is a unique distribution of particles among energy levels. Motion • Three types of motion. Translational Rotational Vibrational • As the temperature of a sample increases, the amount of motion increases. Energy States Statistical Entropy • Entropy is related to the number of microstates by the following equation: S = k ln(W) S is entropy W is the number of microstates* k is the Boltzmann constant (k = 1.38 x 10-23 J/K) *For an ideal gas, W can be counted as permutations in a range of positions and momenta. W = N!/PNi! Ludwig Boltzmann (18441906) founded the field of statistical mechanics and statistical thermodynamics. These ideas pre-suppose the existence of (or the reality of) atoms…which were opposed by the leading physicists of the day, in particular Ernst Mach. Boltzmann turned to philosophy to refute objections to his’ theory, but ultimately committed suicide. Driving the Human Engine • Exergonic reactions are spontaneous (DG < 0). • Endergonic reactions are nonspontaneous (DG > 0). • The laws of thermodynamics describe the chemical reactions that power the human engine. Glycolysis The conversion of glucose to glucose 6-phosphate is the first step in the catabolism (physiological combustion) of starches. The energy for this process is provided by the hydrolysis of ATP ChemTour: Entropy Click to launch animation PC | Mac This ChemTour includes an “Entropy Battle” game that challenges students to maintain order within a system as the temperature rises and the phase level moves from solid to gas. ChemTour: Dissolution of Ammonium Nitrate Click to launch animation PC | Mac ChemTour: Gibbs Free Energy Click to launch animation PC | Mac Students learn to calculate the maximum potential energy available to do work in a system. An interactive “Gibbs free energy calculator” allows students to manipulate variables entropy, enthalpy, and temperature to explore the effect on DG of a reaction. Shown to the left are three possible configurations (A, B, and C) for placing 4 atoms in two boxes. Which of the following processes is accompanied by the largest increase in entropy, ΔS? A) A → B B) B → C Entropy of Four Atoms in Two Boxes C) C → A Consider the following arguments for each answer and vote again: A. The entropy change from state A, which has 1 microstate, to state B, which has 4 microstates, is the greatest. B. State C is the most probable equilibrium state, so the B → C transition has the largest entropy. C. The entropy of the C → A transition is equal to the sum of the entropies for the A → B and B → C transitions. Entropy of Four Atoms in Two Boxes An ideal gas in a sealed piston is allowed to expand isothermally and reversibly against an external pressure of 1.0 atm. What can be said of the change in the entropy of the surroundings, ΔSsurr, for this process? A) ΔSsurr > 0 B) ΔSsurr = 0 Isothermal Expansion of an Ideal Gas C) ΔSsurr < 0 Consider the following arguments for each answer and vote again: A. The gas is doing work, thereby increasing the entropy of the surroundings. B. For a reversible expansion, entropy is constant, so ΔSsys = ΔSsurr = ΔSuniv = 0. C. The expansion is isothermal, ΔSsys > 0, and reversible, ΔSuniv = 0. Therefore, ΔSsurr < 0. Isothermal Expansion of an Ideal Gas An ideal gas is expanded adiabatically (q = 0) into a vacuum. Which of the following statements is true for this process? A) ΔEsys < 0 B) ΔGsys < 0 Gas Expansion into a Vacuum C) ΔSsys < 0 Consider the following arguments for each answer and vote again: A. During the expansion, the gas performs work, so the energy decreases. B. The expansion of a gas into a vacuum is a spontaneous process, so ΔGsys is negative. C. Although the gas volume increases, the temperature decreases dramatically, thereby reducing the entropy. Gas Expansion into a Vacuum Consider the following possible gas phase reaction: Which of the following is probably true for this reaction? A) ΔH > 0 B) ΔS > 0 Formation of CH Cl from CH and CCl C) ΔG > 0 Consider the following arguments for each answer and vote again: A. It is energetically more favorable to have all of the same type of bonds in a molecule than it is to mix and match. B. There are more microstates for the arrangements of H and Cl atoms on 2 CH2Cl2 molecules than on a CH4 and a CCl4 molecule. C. Free energy is required to accommodate formation of the electric dipole moment on CH2Cl2. Formation of CH Cl from CH and CCl What can be said of ΔG° for the condensation of water vapor, H2O(g) → H2O( ), at 25°C if the partial pressure of H2O(g) is 1.0 atm? A) ΔG° > 0 B) ΔG° = 0 ΔG° for Condensation of Water at 25° C C) ΔG° < 0 Consider the following arguments for each answer and vote again: A. The vaporization of water is spontaneous when the partial pressure of H2O(g) is 1.0 atm at 25°C. Therefore, ΔG° > 0 for the condensation of water vapor. B. At 25°C, condensation will occur spontaneously only when the partial pressure of H2O(g) rises above the equilibrium partial pressure of 1.0 atm. C. The equilibrium partial pressure of H2O(g) is less than 1.0 atm at 25°C, so water vapor will condense spontaneously. ΔG° for Condensation of Water at 25° C To the left is a plot of vapor pressure versus temperature for the vaporization of ethanol. C2H5OH(λ) → C2H5OH(g). At which temperature is ΔG° = 0 for the vaporization of ethanol at 1.0 atm? A) > 100°C B) 100°C ΔG° of Vaporization of Ethanol C) < 100°C Consider the following arguments for each answer and vote again: A. Ethanol has a higher molecular mass than water and so requires more heat for vaporization. B. The temperature at which ΔG° = 0 for vaporization is 100°C for all liquids. C. For ethanol, ΔG° = 0 when the vapor pressure equals 1.0 atm, which occurs at a temperature lower than 100°C. ΔG° of Vaporization of Ethanol Which of the following plots shows the correct relationship between ΔG° (yaxis) and temperature (x-axis) for the sublimation of solid iodine to iodine vapor at 1.0 atm? A) B) ΔG° Versus T for the Sublimation of I (s) C) Consider the following arguments for each answer and vote again: A. Once the temperature becomes high enough that the equilibrium partial pressure of I2(g) is greater than 1.0 atm, the reaction will be spontaneous. B. This process becomes less spontaneous at higher temperatures because more iodine must be vaporized. C. As the temperature increases and the system approaches equilibrium, ΔG° will decrease. When the system moves past equilibrium, ΔG° will begin to increase. ΔG° Versus T for the Sublimation of I (s) The formation of ozone, O3(g), from molecular oxygen is an endothermic process, with ΔH° = 85 J/mole. 3 O2(g) 2 O3(g) At what temperatures will the reaction proceed spontaneously if PO2 = PO3 = 1.0 atm? A) High temperatures B) Low temperatures C) No temperatures Spontaneity of Ozone Formation Consider the following arguments for each answer and vote again: A. Because the formation of ozone is an endothermic reaction, it will be spontaneous only at high temperatures. B. The formation of ozone will occur only at low temperatures, where the O2(g) molecules will begin to condense and form O3(g) molecules. C. The reaction is endothermic and ΔS° < 0, so at no temperature can this reaction be spontaneous at 1.0 atm. Spontaneity of Ozone Formation