* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Gene expression wikipedia , lookup
Biochemistry wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein moonlighting wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
Transcript
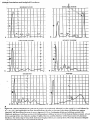
Plasma Proteins Reading assignment Harper’s Biochemistry: PP 737-745 Dr. Zeyad El-Akawi Jreisat, M.D, M.A, Ph.D Blood • Solid elements: – Red cells – White cells – Platelets • Liquid medium: – Plasma Blood Major Functions of Blood - Respiration - Nutrition - Excretion - Acid-base balance - Water balance - Body temperature regulation - Defense - Hormone transport and regulation of metabolism - Metabolite transport - Coagulation Difference between plasma and serum???? Serum = plasma - coagulation factors Plasma Composition • Water • Plasma proteins 92% 7% (total 7.0-7.5 g/dL) – Simple – Conjugated • Glycoproteins • Lipoproteins • • • • • Electrolytes (Na+, K+, Ca+2, Cl-, HCO3-) Metabolites Nutrients Hormones Other solutes 1% Plasma proteins • Most plasma proteins, with the exception of immunoglobulins and protein hormones are synthesized in the liver • Plasma proteins are generally synthesized on membranebound polyribosomes – Rough endoplasmic membrane → smooth endoplasmic membrane → Golgi apparatus → secretory vesicles → Plasma • Almost all plasma proteins are glycoproteins • Plasma proteins circulate in the blood and between the blood and the extra-cellular tissue spaces. Their movement occurs not only by passive diffusion through junctions between capillary endothelial cells but by active transport mechanisms and by pinocytosis and exocytosis Plasma proteins • Because of this movement, most extra-vascular fluids • • • • normally contain small amount of plasma proteins The concentration of protein in the plasma is important in determining the distribution of fluid between blood and tissues Many plasma proteins exhibit polymorphism – Alpha1-antitrypsin, haptoglobin, transferrin, ceruloplasmin and immunoglobulins Each plasma protein has a characteristic half-life in the circulation Most plasma proteins are catabolized in the liver. Plasma proteins • Alterations in plasma proteins occurs in health and disease • The levels of certain proteins in plasma increase during • • acute inflammatory states or secondary to certain types of tissue damage Some of these alterations have genetic origin, many more reflect physiological or pathological processes Variations in the amount or kinds of protein found in plasma or extra-vascular fluids depend on many factors – Genetic – Physiological – Pathological Classification of plasma proteins based on their function • Antiproteases: antichymotrypsin, alpha1-antitrypsin, • • • • • • alpha2-macroglobulin, antithrombin Blood clotting: various coagulation factors, fibrinogen Enzymes: – Function in blood; coagulation factors, cholinesterase – Leakage from cells or tissues; aminotransferases Hormones: Erythropoietin Immune defense: immunoglobulins, complement proteins, beta2-microglobulin Involvement in inflammatory responses: Acute phase response proteins (c-reactive proteins, alpha1-acid glycoprotein) Oncofetal: alpha-1 fetoprotein (AFP) Classification of plasma proteins based on their function • Transport or Binding proteins: – Albumin; various ligands, including bilirubin, free fatty acids, ions (Ca+2), metals (Cu+2, Zn+2), metheme, steroids and other hormones, drugs. – Ceruloplasmin: contains Cu+2 – Corticosteroid-binding globulin: transcortin (binds cortisol) – Haptoglobin: binds extracorpuscular hemoglobin – Lipoproteins: chylomicrons, VLDL, LDL, HDL – Hemopexin: binds heme – Ritenol-binding protein: binds retinol – Sex hormone-binding globulin: binds testosterone, estradiol – Thyroid-binding globulin: binds T3, T4 – Transferrin: transport iron – Transthyretin (prealbumin): binds T4 and forms a complex with retinol-binding protein Plasma proteins separation • Separation of individual proteins from a complex mixture is accomplished by the use of solvents or electrolytes or both to remove different protein fractions in accordance with their solubility characteristics. • Salting out: a method for separation of plasma proteins using various concentrations of [Sodium or ammonium sulfate] • Plasma proteins can be separated by this method into three groups – Fibrinogen – Albumin – Globulins Plasma proteins separation • Electrophoresis: – The most common method of analyzing plasma proteins – Using different supporting medium, the most common in clinical laboratories is cellulose acetate (electrophoretogram) – Separated proteins into five bands: albumin, alpha1, alpha2, beta, and gamma fractions – The amount of bands quantified by densitometric scanning machine Electrophoresis Plasma protein electrophoresis Quantified of plasma proteins by densitometric scanning machine Plasma proteins separation • Antibodies: – Specific plasma proteins are separated by specific monoclonal antibodies fixed on stationary phase (Column Chromatography) – Allowing isolation of pure proteins from the complex mixture present in plasma Acute phase reactant proteins **Concentration of these proteins rise significantly in acute inflammation, chronic inflammation and cancer** • Alpha1-antitrypsin (AAT): congenital deficiency may be associated with emphysema or cirrhosis • Alpha1-acid glycoprotein (AAG): binds cationic drugs and hormones • Haptoglobin (HAP): binds hemoglobin, reduced by hemolysis • Ceruloplasmin (CER): contains copper, antioxidant, decreased in Wilson’s disease • C4: Complement factor • C3: Complement factor • C-reactive protein (CRP): Nonspecific defense against infectious agents • Fibrinogen **Stimulatory factors, Interleukin-1 (IL-1) and interleukin-6 (IL-6) at the gene level** Plasma proteins • Albumin: – is the major protein of human plasma (3.4-4.7 g/dL) – Approximately 40% of albumin is present in plasma and the other 60% in the extracellular space – It synthesized in the liver as preproprotein – The synthesis of albumin is depressed in a variety of diseases, particularly those of the liver (decreased albumin/globulin ratio) – Responsible for 75-80% of the osmotic pressure of human plasma – Absence of albumin (analbuminemia) might caused by mutation that affect splicing – It binds many ligands (free fatty acids, calcium, certain steroid hormones, bilirubin, tryptophan) – It binds and transport drugs (sulfonamides, penicillin G, dicumarol, aspirin) – It transports copper • Haptoglobin: – Binds extracorpuscular hemoglobin preventing free hemoglobin from entering the kidney – Exist in three polymorphic forms, Hp1-1, Hp2-1, Hp2-2 – Low levels of haptoglobin are found in patients with hemolytic anemias – It is an acute phase protein and its plasma level is elevated in a variety of inflammatory states • Transferrin: – – – – – Is a beta-1 globulin It is a glycoprotein synthesized in the liver Shuttles iron to sites where it is needed Transferrin diminishes the potential toxicity of iron The concentration of transferrin in plasma is approximately 300 mg/dL that can bind 300 µg of iron per deciliter (total iron-binding capacity) of plasma • Ferritin: – Normally there is a little ferritin in human plasma – In patients with excess iron, the amount of ferritin in plasma is markedly elevated – Index of body iron stores – Synthesis of the transferrin receptors and that of ferritin are reciprocally linked to cellular iron content – Iron response elements – Iron-responsive element-binding protein • Hemosiderin: – Partly degraded form of ferritin but still containing iron • Primary hemochromatosis: is a common genetic disorder characterized by excessive storage of iron in tissues leading to tissue damage • Secondary hemochromatosis: can occurs in the result of increased iron levels by transfusion, intake, hemolysis • Ceruloplasmin: – – – – It is an alpha-2 globulin Binds copper Low levels of this protein are associated with Wilson disease It exhibits a copper-dependent oxidase activity • Copper: – Is a cofactor for certain enzymes including, amine oxidase, copperdependent superoxide dismutase, cytochrome oxidase, tyrosinase – It is excess can cause problems because it can oxidize proteins and lipids, bind to nucleic acids and enhance the production of free radicals – Metallothioneins: are a group of small proteins found in the cytosol of cells particularly of liver, kidney, and intestine they control of copper levels • Menkes Disease: “kinky” or “Steely” hair disease – Is due to mutations in the gene for a copper-binding P-type ATPase leads to abnormality in copper metabolism – X-linked – Defect in copper exit from cells leads to its accumulation – Involves the nervous system, connective tissue and vasculature • Wilson Disease: – Is due to mutations in the gene for a copper-binding P-type ATPase results in the failure of copper to be excreted in the bile – Copper toxicoses • Alpha-1 antitrypsin deficiency: a serine protease inhibitor – Is associated with emphysema and liver disease – Synthesized by hepatocytes and macrophages