* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 22-Lutz
Survey
Document related concepts
Proteolysis wikipedia , lookup
MTOR inhibitors wikipedia , lookup
Biosynthesis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Drug design wikipedia , lookup
Butyric acid wikipedia , lookup
Lipid signaling wikipedia , lookup
Biochemistry wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Transcript
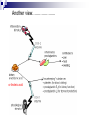
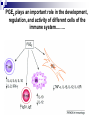
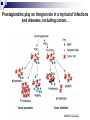
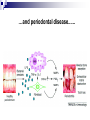
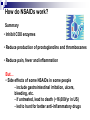
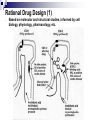
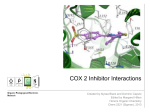
Prostaglandins: Synthesis, functions and inhibitors Carol S. Lutz, Ph.D. Lecture 23 March 16, 2015 Objectives: •Discover and distinguish relationships between different types of eicosanoid molecules (PGs, TXs, LTs) •Gain knowledge of enzymes that create PGs and TXs (COX-1 and -2) •Explore physiological functions of these molecules •Ascertain how pharmacological inhibitors work to block activity Prostaglandins and related compounds (thromboxanes and other eicosanoids): - Potent cell signalling molecules – paracrine hormones - Multiple effects, including pain and inflammation associated with arthritis Synthesized from essential dietary fatty acids, esp. linoleic acid Prostaglandins: -discovered in the 1930s by Ulf von Euler -synthesized in virtually every cell in the body -unsaturated 20 carbon molecules, also contain a 5-member ring -work right within the cells where they are synthesized—”act locally” -have an extremely short half-life and are not stored Examples of prostaglandin structures -note the similarities and differences Lippincott Fig 17.21 The first step in the synthesis of prostaglandins is the oxidative cyclization of free arachidonic acid to yield PGH2 by prostaglandin endoperoxide synthase. PGH2 is converted to a variety of prostaglandins and thromboxanes by a variety of cell-specific synthases. Note: Two catalytic activities Lippincott 17.22 Cyclo-oxygenase Isoforms (COX-1 vs COX-2) Two isoforms of COX – COX-1 is constitutive, expressed in most tissues – – – Both produce prostaglandins (PGE2, PGF2a, PGI2) physiological and homeostatic role, cell signalling not influenced by steroid administration not increased by cytokines nor bacteria COX-2 is inducible following inflammation, trauma, etc – – – – found in immunocompetent cells (e.g. leukocytes) pathophysiological role, maintains inflammation induced by cytokines (interleukin-1) inhibited by steroids THE COX GENES, mRNAs, PROTEINS The pathway of prostaglandin production, revisited, with insights into pro-inflammatory regulation Another view……………….. or linoleic acid PGE2 plays an important role in the development, regulation, and activity of different cells of the immune system…….. Prostaglandins play an integral role in a myriad of infections and diseases, including cancer… …and periodontal disease...... Functions of prostaglandins 1. Activation of the inflammatory response, production of pain, and fever. When tissues are damaged, white blood cells are mobilized to the site to minimize tissue destruction. Prostaglandins are produced as a result. 2. Blood clots form when a blood vessel is damaged. Closely related molecules called thromboxanes stimulate constriction and clotting of platelets. Conversely, PGI2 is produced to have the opposite effect on the walls of blood vessels where clots should not be forming. Functions of prostaglandins, cont. 3. Certain prostaglandins (i.e. PGE2) are involved with induction of labor by inducing uterine contractions. 4. Prostaglandins are involved in several other organs -regulate salt and fluid balance in body -increase blood flow in kidneys -increase secretion of protective mucus in GI tract -inhibit acid synthesis in GI tract -leukotrienes, related molecules, promote constriction of bronchi associated with asthma The inhibitors…. How do NSAIDs work? Summary • Inhibit COX enzymes • Reduce production of prostaglandins and thromboxanes • Reduce pain, fever and inflammation But… • Side effects of some NSAIDs in some people - include gastrointestinal irritation, ulcers, bleeding, etc. - if untreated, lead to death (~16,000/yr in US) - led to hunt for better anti-inflammatory drugs Effects of aspirin and other pain killers? Aspirin works on both COX-1 and COX-2 to inhibit arachidonic acid’s entry into the active site of the enzyme --acetyl group of aspirin binds to serine in COX -- by blocking the activity of the COX enzymes, this relieves some of the effects of pain and fever --”nonselective” --many side effects Tylenol—thought to have effects through inhibiting the activity of COX-3, an alternatively spliced form of COX-1 Low dose aspirin therapy: -Aspirin irreversibly inhibits COX-1 (and COX-2) -Has a short half-life -New platelets are constantly being made -Will therefore reduce but not abolish the ability for blood to clot, thereby reducing heart attacks and stroke Short History of COX-2 Inhibitors (1) • 1970’s - aspirin target identified as cyclo-oxygenase (COX) • 1980’s - Two forms of COX identified: COX-1, constitutive COX-2, induced at sites of inflammation - Hypothesis: selective inhibition of COX-2 might reduce inflammation without the GI side effects. • 1990’s - Rational design of COX-2 inhibitors Rational Drug Design (1) - Based on molecular and structural studies, informed by cell biology, physiology, pharmacology, etc. Rational Drug Design (2) Non-selective NSAIDs COX-1 COX-2 Selective COX-2 inhibitors COX-2 inhibitors Coxibs – a new class of NSAIDs Merck Pfizer Vioxx (rofecoxib) – withdrawn from sale by Merck, September 30, 2004 Arcoxia (etoricoxib) – successor to Vioxx? Not (yet) approved in US Celebrex (celecoxib) – FDA recommended ‘black box’ warning label Bextra (valdecoxib) –withdrawn from the market, 2005 Short History of COX-2 Inhibitors (2) • mid 1990’s - X-ray crystal structures showed that COX-2 active site has a side pocket that is absent in COX-1. • late 1990’s - inhibitors designed that preferentially bind COX-2, are equally effective as NSAIDs, but are reported to cause less GI damage. • 1998/1999 - Celebrex and Vioxx approved by FDA for osteoarthritis and rheumatoid arthritis. • 2000’s - next generation of drugs developed with even higher selectivity for COX-2 over COX-1. Triumph for rational drug design Freedom from GI side effects make the COX-2 inhibitors very attractive……. Short History of COX-2 Inhibitors (3) How the problems came to light June 2000-VIGOR trial-compared Vioxx to naproxen-could not tell if Vioxx was bad or naproxen was protective Sept. 30, 2004 - APPROVe trial (Adenomatous Polyp Prevention on Vioxx) led Merck to withdraw Vioxx voluntarily. Randomized, placebo-controlled trial (2606 patients, for Vioxx use in preventing colorectal polyps) showed an increased risk of heart attack and stroke. The increased risk is now estimated as 2.3-fold (from a meta-analysis). Dec. 20, 2004 - APC trial (Adenoma Prevention by Celecoxib) by Pfizer reported ~ 2.5-fold increase in major fatal or non-fatal cardiovascular events. Summary •Prostaglandins, thromboxanes and leukotrienes are known as eicosanoids •They are produced in small amounts in almost all tissues, act locally, and have many important physiological and pathological functions •The dietary precursor of eicosanoids is the essential fatty acid, linoleic acid •Synthesis of prostaglandins begins with oxidative cyclization of free arachidonic acid to yield PGH2 by prostaglandin endoperoxide synthase (cyclooxygenase) •There are two isozymes of the synthase: COX-1 and COX-2, which are important drug targets