* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Setting the stage
Metastable inner-shell molecular state wikipedia , lookup
Metallic bonding wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Computational chemistry wikipedia , lookup
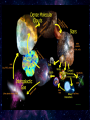
Cosmic dust wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Polycyclic aromatic hydrocarbon wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Molecular dynamics wikipedia , lookup
Analytical chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Nuclear magnetic resonance spectroscopy wikipedia , lookup
Abiogenesis wikipedia , lookup
Chemical bond wikipedia , lookup
Biochemistry wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Utilization of spectroscopy in the determination of the origins of life Helgi Rafn Hróðmarsson Friday seminar 28.03.14 Overview Setting the stage „In the beginnning...“ The spectroscopy „He saw the light was good and separated it from darkness.“ The implications “Then the eyes of both of them were opened.” Overview Setting the stage „In the beginnning...“ The spectroscopy „He saw the light was good and separated it from darkness.“ The implications “Then the eyes of both of them were opened.” 4 1 1𝐻 1 1𝐻 → + 2 1𝐻 1 1𝐻 + 3 2𝐻𝑒 12 6𝐶 16 8𝑂 13 7𝑁 → 4 2𝐻𝑒 + → 1 1𝐻 2 1𝐻 → + 3 2𝐻𝑒 16 8𝑂 13 6𝐶 13 7𝑁 + + 2𝜈𝑒 + 2𝛾 𝑒+ → + 𝜈𝑒 15 7𝑁 +𝛾 1 1𝐻 → 16 8𝑂 → +𝛾 + 𝑒+ 12 6𝐶 12 6𝐶 + 42𝐻𝑒 → 17 8𝑂 7 3𝐿𝑖 + 11𝐻 → 85𝐵 + 𝛾 20 10𝑁𝑒 + 11𝐻 → + 𝜈𝑒 + 126𝐶 → 12 6𝐶 17 9𝐹 +𝛾 14 7𝑁 +𝛾 3 2𝐻𝑒 + 11𝐻 → 2 42𝐻𝑒 + 42𝐻𝑒 → 74𝐵𝑒 + 𝛾 → 84𝐵𝑒 + 𝑒 + + 𝜈𝑒 +𝛾 8 4𝐵𝑒 + 42𝐻𝑒 13 6𝐶 + 42𝐻𝑒 → + 11𝐻 → + 42𝐻𝑒 7 5𝐵 8 4𝐵𝑒 15 7𝑁 16 8𝑂 + 𝑒 − → 73𝐿𝑖 + 𝜈𝑒 + 11𝐻 → 7 3𝐵𝑒 16 8𝑂 +𝛾 + 𝑒 + + 𝜈𝑒 + 42𝐻𝑒 ↔ 84𝐵𝑒 7 4𝐵𝑒 24 4 12𝑀𝑔 + 2 2𝐻𝑒 28 4 14𝑆𝑖 + 2𝐻𝑒 31 + 15𝑃 + 𝑝 15 8𝑂 15 7𝑁 4 2𝐻𝑒 + 32𝐻𝑒 → 42𝐻𝑒 + 2 11𝐻 + 11𝐻 → + 2𝑒 + + 11𝐻 → 12 6𝐶 → 2 42𝐻𝑒 14 7𝑁 +𝛾 + 42𝐻𝑒 → 16 8𝑂 16 4 15 14 1 8𝑂 + 2 2𝐻𝑒 7𝑁 + 1𝐻 → 8𝑂 + 𝛾 20 4 10𝑁𝑒 + 2𝐻𝑒 17 17 + 23 + 9𝐹 → 8𝑂 + 𝑒 + 𝜈𝑒 11𝑁𝑎 + 𝑝 +𝛾 Overview Setting the stage „In the beginnning...“ The spectroscopy „He saw the light was good and separated it from darkness.“ The implications “Then the eyes of both of them were opened.” 1968 – Interstellar NH3.1 1969 – Interstellar H2O vapour2 & H2CO.3 1970 – Interstellar CO.4 1Cheung, A. C., Rank, D. M. Townes, C. H., Thornton, D. D., and Welch, W. J. 1969, Phys. Rev. Letters. 21, 1701. 2Cheung, A. C., Rank, D. M., Townes, C. H., Thornton, D.D. And Welch, W. J. 1969, Nature. 221, 626. 3Snyder, L.E., Buhl, D., Zuckerman, B., and Palmer, P. 1969, Phys. Rev. Lett., 22, 679. 4Wilson, R. W., Jefferts, K. B. And Penzias, A. A. 1970, Astrophys. J. Lett. 161, L43. White, G. J., Araki, M., Greaves, J. S., Ohishi, M., and Higginbottom, N. S. 2003. A&A, 407, 589. Walmsley, C. M., & Ungerecths, H. 1983, A&A, 122, 164. Infrared spectroscopy What if we compile all of the interstellar and circumstellar molecules that have been detected with these spectroscopic techniques? Well... According toWikipedia... 43 Diatomics, 41 triatomics, 26 with four atoms, 19 with five atoms, 15 with six atoms, 9 with seven atoms, 10 with eight atoms, 9 with nine atoms, 15 with ten or more atoms and 17 deuterated molecules. 204 different molecules altogether who‘s ingredients include H, C, N, O, F, Na, Mg, Al, Si, P, S, Cl, K, Ti, Fe, I. „Mixtures of free molecular PAHs dominated by PAH ions, can account for the global appearance of interstellar emission spectra and the variations of those spectra.“ Then what??? -Allamandola & Hudgins. Solid State Astrochemistry. 2000, pp.263. THE NASA Ames PAH Spectral Database 580 theoretically computed and 200 laboratory measured spectra of PAHs ranging in size from C10H8 to C130H28. Spectra of PAHs in neutral and singly charged (+/–) states. Spectra of a few multiply charged species also included. www.astrochem.org/pahdb -Allamandola & Hudgins. Solid State Astrochemistry. 2000, pp.265-267. H2O:CH3OH:NH3: CO:C3H8 (100:50:10:10:10) interstellar ice analogs as traced by infrared spectra measured at 10 K. Note the ready formation of CO2, H2CO, CH4 and XCN at the expense of CH3OH. Allamandola, L. J., Sandford, S. A., and Valero, G. (1988), Icarus, 76, 225. Bernstein, M. P., Sandford S. A., Allamandola L. J., Chang S., and Scharberg M. A. (1995), Ap. J., 454, 327. Interstellar/Precometary Ice Photolysis: Abiotic synthesis of Important Prebiotic Organics N-Formyl glycine All the necessary Cycloserine components to make an RNA world. Sugar precursors Amino acids Molecules capable of vesicle formation. Hydantoin Amino malonic acid Urea Ethanolamine Serine Glyeric Acid Glycerol Bernstein, et al.(2002), Nature, 416, 401. Deamer, D. W. Evolution: Ivestigating the Evidence, Paleontological Society Special Publication. 1999, 9. Deamer, D. W. 2012, Chem. Soc. Rev., 2012, 41, 5375. Overview Setting the stage „In the beginnning...“ The spectroscopy „He saw the light was good and separated it from darkness.“ The implications “Then the eyes of both of them were opened.” Conclusion Microwave and infrared spectroscopy have been used to detect a wide variety of interstellar molecules and illuminate properties of their stellar environments. Infrared spectroscopy utilized in chemical simulations of reactions on interstellar ices. Various studies have been performed on amphiphilic vesicle forming molecules. Thank you for your attention!